In order to reduce dependence on fossil chemicals and to achieve the net zero targets, lignin based polyurethanes (LPU) are most desirable

Achieving net zero emissions by mid-century necessitates the deployment of Carbon Capture, Utilization, and Storage (CCUS) technologies across industrial sectors, particularly those with inherently high process emissions such as cement, chemicals, and steel. CCUS enables the direct removal of CO₂ from point sources or the atmosphere and its subsequent sequestration or conversion into value-added products.
In the chemical and polymer industries, a parallel shift toward renewable feedstocks is underway, with biomass emerging as a viable and sustainable alternative to fossil-derived raw materials. Renewable biomass, when processed into platform chemicals like bio-ethylene, succinic acid, and lactic acid, can serve as the basis for producing bio-polymers such as polyethylene, polybutylene succinate, and polylactic acid. Integrating biomass valorization with CCUS technologies offers a synergistic route to not only sequester carbon but also to create polymers with inherently lower life-cycle greenhouse gas emissions. Moreover, this integration supports the broader development of a circular carbon economy, wherein carbon is continuously recycled and reused, thus minimizing net atmospheric additions. By coupling CCUS, renewable energy, and bio-based feedstocks, the polymer sector can significantly contribute to decarbonization targets while fostering sustainable material innovation.
CCUS AND Polyurethanes
CCUS is increasingly being integrated into the production of polyurethanes by utilizing captured CO₂ as a raw material for polymer synthesis. Traditionally, polyurethanes are manufactured by reacting polyols (compounds with multiple hydroxyl groups) with isocyanates. Recent advancements allow captured CO₂ to be chemically incorporated into polyol structures through catalytic processes, producing CO₂ -based polyols that can replace up to 20–40 per cent of the conventional fossil-derived content. Companies such as Covestro have commercialized such technologies, developing CO₂ -containing polyether carbonate polyols that maintain desirable polyurethane properties for use in foams, coatings, adhesives, and elastomers. This approach not only reduces the carbon footprint of polyurethane products but also transforms captured CO₂ into durable materials, embodying the principles of carbon circularity and advancing net zero goals.
Importance of Polyurethane
Polyurethane (PU), renowned for its exceptional versatility, is a well-known polymer widely utilized in elastomers, foams, sealants, coatings, fibres, adhesives, and a myriad of other applications, spanning automotive parts, clothing, biocompatible medical products, and interior/exterior finishing materials for housing. Polyurethanes (PUs) are characterized by urethane linkages (–NH–(C=O)–O–), formed through the polyaddition reaction of a polyol with di- or poly-isocyanate (–N=C=O) compounds. PUs can be tailored to various densities, morphologies, and stiffnesses, making them ideal for applications in several industries. Within the PU product spectrum, foams dominate, categorized as rigid, semi-rigid, or flexible based on core densities, stiffness, and mechanical properties. The global polyurethane foams market is anticipated to grow at a 7.6 per cent CAGR, from US$ 42.4 billion in 2023 to US$ 62.5 billion by 2030 during the forecast period 2025-2030 as per the report of Vynz Research.
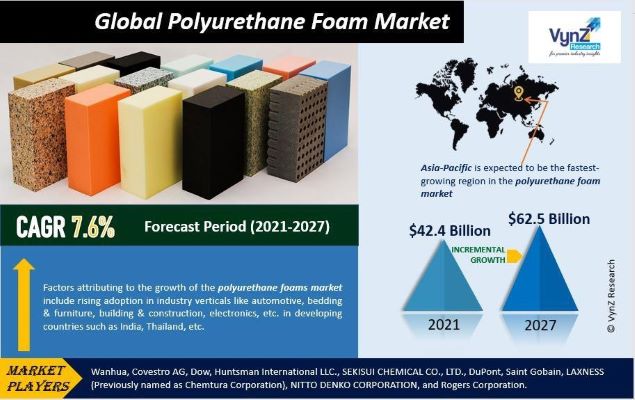
Figure 1. PU market (Source: Vynz Research market survey)
Polyol, a primary component of PU foams, typically constitutes 30–70 wt. per cent depending on the foam's intended use. Presently, most PU foams rely on fossil-fuel-based polyols for production. Polyurethanes (PUs) are synthesized through the addition reaction of polyols with terminal OH groups and diisocyanates (or polyisocyanates), forming PU (carbamate) groups within the polymer backbone, as depicted in Scheme 1.
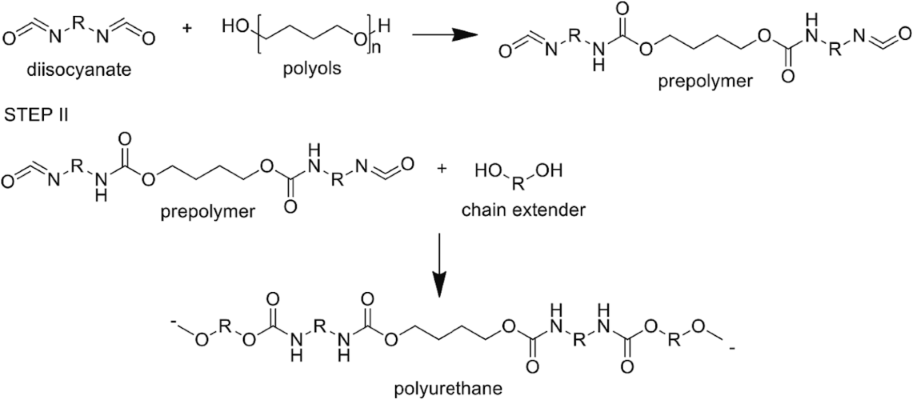
Scheme 1. Synthesis of PU
In the context of depleting fossil fuel reserves and the net zero emissions of carbon dioxide by 2050, the use of biomass for production of chemicals, fuels, polymers and materials assumes great significance. Since lignin contains several hydroxyl groups, it can be used to substitute the petroleum derived polyols. The utilization of natural and renewable resources such as lignin, predominantly comprising phenylpropanoid with abundant hydroxyl (OH) groups, phenols, and carbonyls, holds promising potential for replacing petroleum-based polyols in PU synthesis.
Lignin as a Feedstock
Roughly 2 per cent of the 70 million tons of lignin produced annually is incinerated to substitute fossil resources and generate heat in pulping mills. However, given its inherent traits such as low toxicity, eco-friendliness, biocompatibility, and susceptibility to enzymatic breakdown, lignin presents itself as a promising candidate for the production of high-value goods rather than mere combustion. Traditionally relegated to low-value fuel, lignin exhibits tremendous resistance to digestion compared to other naturally occurring substances. Nonetheless, lignin and its derivatives are increasingly recognized for their potential as value-added products in various large-scale applications.
Isolated lignin presents promising potential for the production of high-value goods, including low-cost carbon fibre, engineering plastics, thermoplastic elastomers, polymeric foams, membranes, and a spectrum of fuels and chemicals currently sourced from petroleum (Figure 2). However, to be viable, these lignin-derived coproducts must offer competitive pricing while meeting the performance standards of their petroleum-based counterparts. Each product category poses its own unique set of challenges.
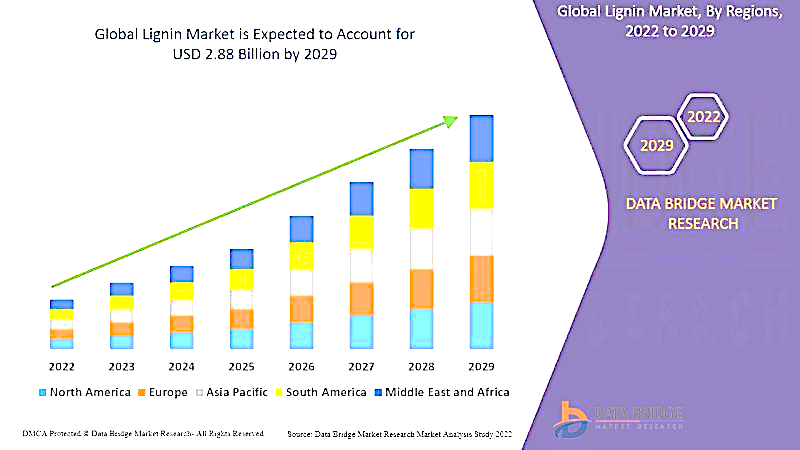
Figure 2. Global lignin market from 2022-2029. Lignin market is expected to be 2.88 billion US$ by 2029. (Source: https://www.databridgemarketresearch.com/reports/global-lignin-market)
As the most abundant aromatic biopolymer, lignin’s structure is rich in carbon, comprising over 60 per cent of its composition. The advancement of renewable lignin-based polymers hinges on the enhancement of processing technologies and the integration of tailored bioenergy crops that yield lignin with desired chemical and physical attributes. In the realm of fuels and chemicals, various approaches for lignin depolymerization and upgrading have emerged, encompassing thermochemical treatments, as well as homogeneous and heterogeneous catalysis. Lignin serves to bind cellulose and hemicellulose fibres together, providing structural support and shielding plants from microbial degradation. The proportion of lignin varies depending on the plant source, with softwood (SW) containing approximately 25–39 per cent lignin, hardwood (HW) about 20–25 per cent, and grasses typically ranging from 15–25 per cent.
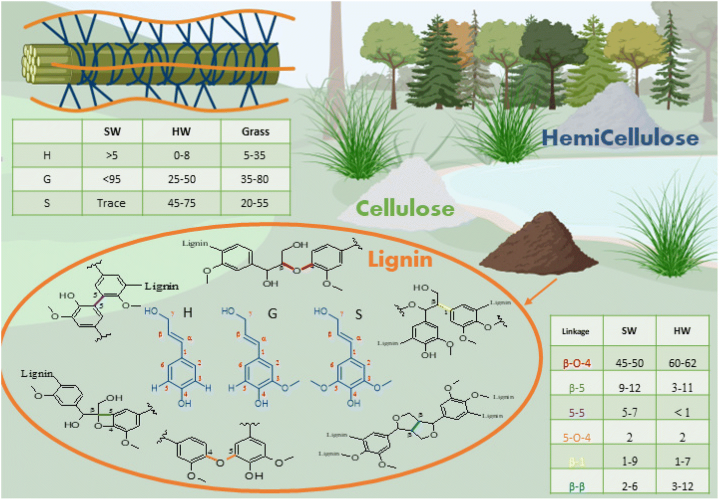
Figure 3. Lignin as a source of chemicals and materials (source: DOI: 10.1039/D2GC02724K, Green Chem., 2022, 24, 8193-8226). SW=soft wood, HW=hard wood, Different constituent structures H, G, and S in lignin
Variations in physical properties arise from differences in synthesis methods and constituent materials. Given its abundance of aliphatic and phenolic hydroxyl functionalities, lignin can readily substitute the macropolyol component of PUs. Moreover, PU derived from petroleum-based polyols exhibits lower biodegradability compared to lignin-based counterparts.
The biologically enhanced properties of chemically modified lignin exhibit superior antibacterial, antioxidant, biocompatible, flame-retardant, hydrophobic, and UV-blocking attributes, likely attributable to the introduction of new functionalities. In recent years, there has been a significant shift towards utilizing renewable resources in the development of polymeric materials. This movement has garnered considerable attention as an alternative to petroleum-based products, with a focus on plastics and biodegradable polymers sourced from natural and renewable origins. The adverse environmental impact of conventional fossil fuels has prompted a transition towards renewable resources, among which lignin and its derivatives play a prominent role. This strategic shift not only fosters environmental sustainability but also promotes national security, rural economic development, and the mitigation of environmental challenges. Because of the presence of several functionalities in lignin, it is a great source of chemicals, fuels and materials (Figure 4).
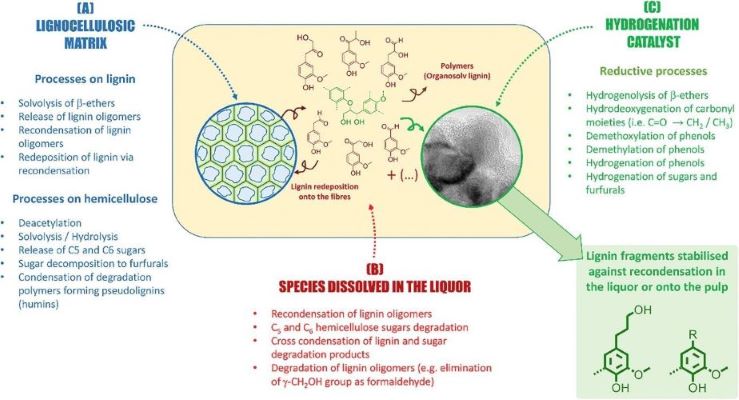
Figure 4. Lignin biorefinery
(Source: DOI:https://doi.org/10.1016/j.joule.2017.11.001)
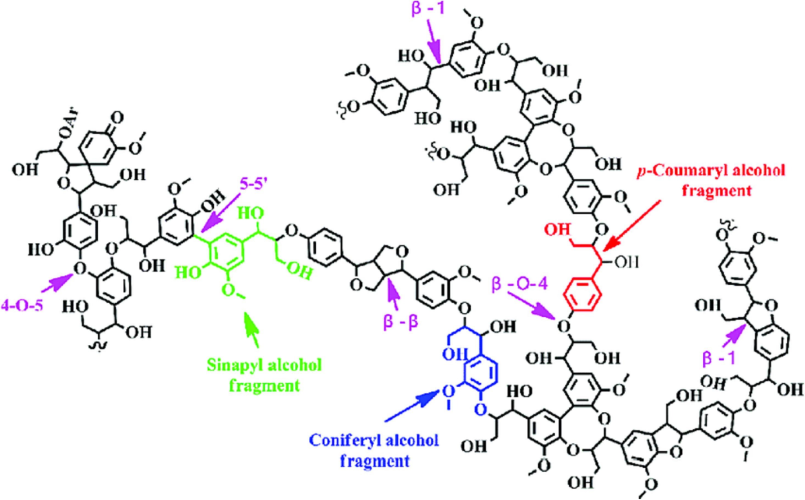
Fig. 5. Schematic model of lignin structure and its major constituents/fragments (sinapyl alcohol, coniferyl alcohol, and p-coumaryl alcohol)
Hydrogenation/hydrogenolysis and oxidation will play a great role in conversion of lignin to a variety of products (Figure 6).
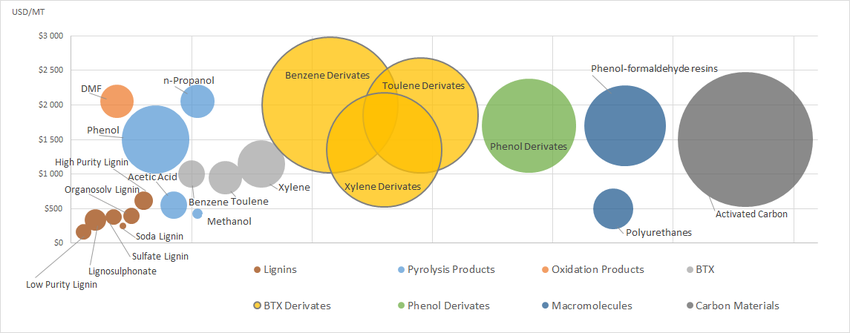
Figure 6. Lignin to chemicals (Source: Wood Research 60(6):973-986 (Dec 2015)) 7, 11, 1831-1837)
LIGNIN PROCESSING
Advancements in wood processing, papermaking, and textile technologies have facilitated the production of lignin and its derivative polymers. These polymers find application in bio-refineries, where they contribute to the generation of a diverse range of products including power, chemicals, materials, and fuel. The majority of lignin and its derivative polyols are sourced from vegetable oils. Vegetable oil, a triglyceride resulting from the combination of glycerol with unsaturated or saturated fatty acids, serves as the primary precursor. A variety of vegetable oils, such as soybean oil, castor oil, rapeseed oil, and bio-glycerol, find application in PU production. Moreover, there exist diverse methodologies for modifying vegetable oils, including oxypropylation, ring-opening, epoxidation, catalytic hydrogenation, and thiolene reaction (also known as alkene hydrothiolation). However, it is worth noting that vegetable oil (excluding castor oil) is also utilized in this regard.
Lignin has long been recognized as a promising source of aromatic compounds; however, traditional lignin depolymerization methods often yield complex mixtures of products. An alkaline aerobic oxidation technique efficiently converts lignin extracted from poplar wood into a range of oxygenated aromatics, including commercially valuable compounds like vanillin and p-hydroxybenzoic acid. The use of centrifugal partition chromatography (CPC) proves effective in isolating individual compounds from the intricate product mixture (Figure 6).
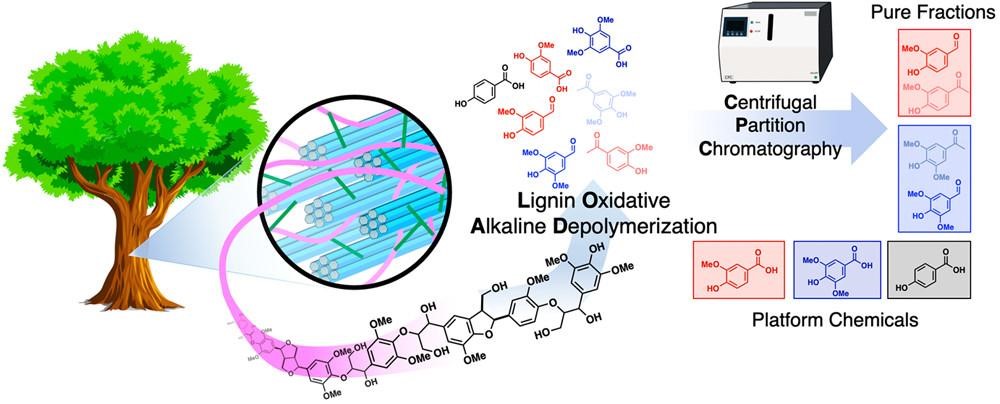
Figure 7. Lignin oxidative alkaline depolymerization (Source: ACS Cent. Sci. 2021, 7, 11, 1831–1837)
Delignification
For industrial applications, the isolation of lignin from lignocellulosic raw materials can be achieved through both chemical and mechanical methods. These processes fall into two main categories: (i) techniques that involve the removal or solubilization of hemicellulose and cellulose, leaving lignin as an insoluble residue; (ii) methods that target the removal or dissolution of lignin, leaving behind the polysaccharides.
The lignin obtained from these processes exhibits variations in size, structure, molecular weight, and physical or chemical properties, influenced by the diverse plant species used and the range of delignification techniques available. Among the most common delignification methods are (a) kraft, (b) sulfite, and (c) organosolv processes, while alternative approaches such as (d) pyrolysis, (e) enzymatic hydrolysis, (f) steam explosion, (g) alkaline hydrolysis, and (h) acidolysis offer less commercial but viable options. Key system parameters such as pH, chemical concentration, temperature, and pressure play critical roles in determining lignin functionality, extraction yield, solubility, and molecular weight, etc.
Lignin based PU
Various methodologies for manufacturing lignin-based polyurethane (LPU), including alkali treatment, oxidation methods, prepolymer techniques, haloalkane modification, and alkane depolymerization, are elucidated in literature. Presently, lignin-based polyurethanes exhibit promising applications across diverse sectors including construction, automotive industries, packaging, textiles, footwear, sporting goods, automobiles, printing rollers, sealants, and binders (Scheme 2).
The inherently multifaceted nature of lignin historically necessitates intricate separation and purification processes to isolate distinct product streams. However, engineering plant feedstocks to achieve greater structural uniformity and tailored functionality holds promise for mitigating these challenges.
Moreover, carbohydrates derived from lignin and its derivatives offer a wide array of applications. These include the synthesis of polyols, polyurethanes (PUs), acrylics, esters, epoxy resins, and phenolic resins featuring aromatic structures, methoxy, alcoholic hydroxyl, phenolic hydroxyl, and other active functional groups. Incorporating lignin into PU production not only reduces reliance on fossil reserves but also enhances the mechanical properties of PU and introduces additional functional characteristics such as flame retardancy, UV protection, and hydrophobicity (Scheme 3).
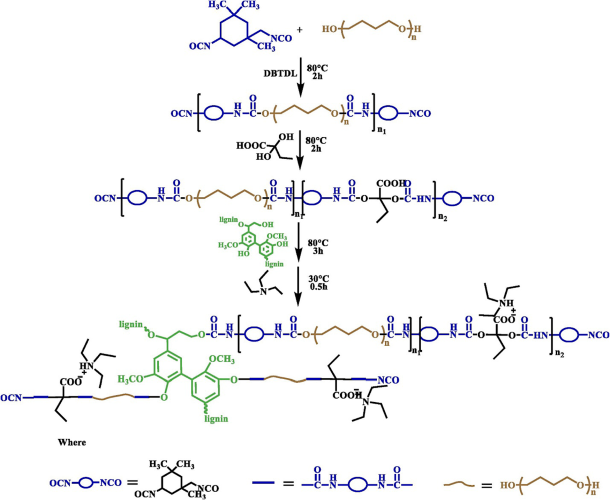
Scheme 2. Lignin-based polyurethane elastomer synthesis
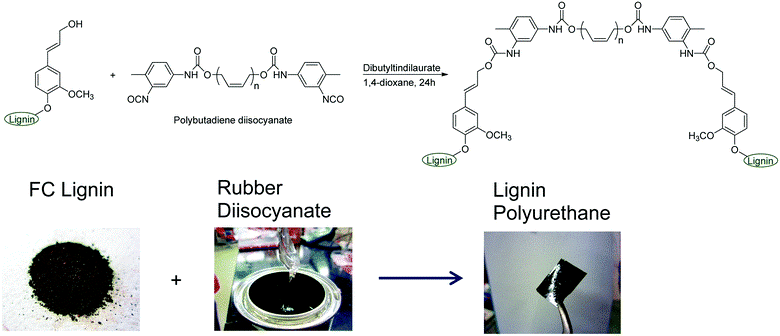
Scheme 3. Lignin-based PU thermoplastic (Source: RSC Adv., 2013,3, 21832-21840).
Polyurethane Synthesis
Thermoplastic polyester can be made by reacting lignin with carboxyl-terminated polybutadiene. Lignin interacts with diisocyanate-terminated polybutadiene to create PU after being cross-linked with formaldehyde to increase uniformity. The soft segment is made of polybutadiene, and the cross-linking point is made of lignin. This leads to the formation of a two-phase network structure and consequently, a thermoplastic polyurethane (TPU) material is developed. TPU elastomer is a material with a lot of applications since it can be manufactured and utilized repeatedly with no environmental impact. Dynamic covalent bonds are also discovered to be useful for recycling cross-linked polymers. Trans-esterification reactions occur with DBTDL as the catalyst, allowing the elastomer to reconstruct at 160◦C under hot pressing. The trans-esterification reaction is slower than usual dynamic bonding and demands the use of a catalyst. Although thermoplasticity achieved through molecular chain motion doesn’t need a catalyst, it can only modify the shape of a material but not repair it. As a result, it would be more beneficial if we could integrate the two mechanisms.
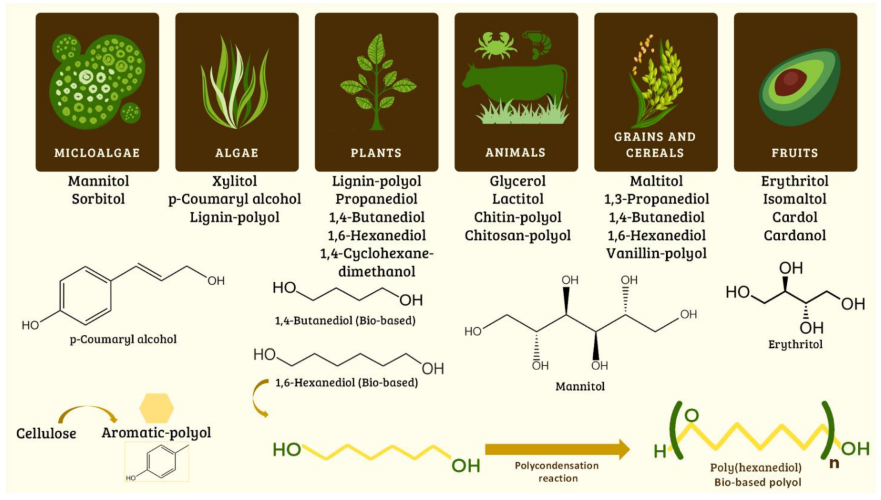
Figure 8. Synthesis of bio-based diols and polyols from natural and renewable resources used to synthesize polyurethanes.(Source: Molecules. 2024 Jan; 29(2): 387.)
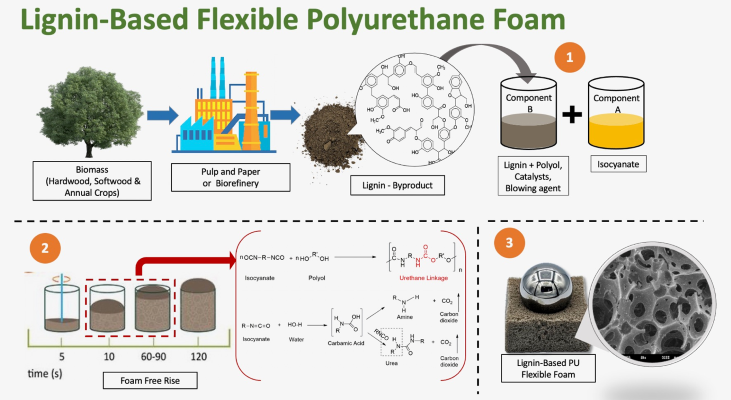
Figure 9. Lignin-based flexible PU foam (Source: ACS Cent. Sci. 2021, 7, 11, 1831–1837)
Thermoplastic Lignin-based Polyurethane Elastomer (TLPUE): Thermoplastic polyester can be synthesized by combining lignin with carboxyl-terminated polybutadiene. The interaction of lignin with diisocyanate-terminated polybutadiene, followed by cross-linking with formaldehyde to enhance uniformity, results in the formation of polyurethane (PU). In this formulation, the soft segment comprises polybutadiene, while lignin serves as the cross-linking point, leading to the development of a two-phase network structure and the creation of a thermoplastic polyurethane (TPU) material.
Lignin-based polyurethane biofoam dressing: Silver nanoparticles have garnered considerable attention for their commercial use as essential biomedical materials. However, their widespread application poses a threat to both environmental ecosystems and human health. To address this concern, silver nanoparticles in material formulations have been partially replaced with naturally occurring antibacterial polylysine (PL). Consequently, a variety of lignin-based polyurethane (LPU) composite biofilms with different formulations have been devised. These biofilms demonstrate rapid development, exhibiting antimicrobial activity against both gram-positive and gram-negative bacteria.
Flame retardant lignin-based PU foam: A three-step procedure to modify lignin with phosphorus, a flame-retardant component, has been reported resulting in the creation of halogen-free, flame-retardant hard PU foam. Flexible PU foams with flame-retardant characteristics have been created by combining phosphorus-containing polyols, lignin, or layered double hydroxide (LDH). Combining lignin, phosphorus-containing polyol, and LDHs resulted in high-viscosity melts that prevented dripping during combustion.
Flame-retarding PU composites (FLPU films): This process lays the groundwork for developing lignin-based flame retardants. The condensation reaction between the hydroxyl (OH) groups of lignin, PEG 2000, polyol 204, or NCO in methyl diisocyanate (MDI) effectively yields polyurethanes (PUs). Additionally, PEG2000 serves to modulate the flexibility of PU segments, further enhancing the versatility of the resulting materials.
Lignin-containing polyurethane adhesives: Their appeal lies in their low toxicity, excellent thermo-stability, rapid curing, superior mechanical properties, and resistance to chemicals and moisture. DBTDL serves as a catalyst in the hydroxymethylation and copolymerization of tolune diisocyanate (TDI) or lignin to produce LPU adhesives.
Lignin-based composites and 3D printing: Thermoplastic materials, including polyhydroxy butyrate (PHBs), polylactic acid (PLA), polyethylene (PE), and acrylonitrile butadiene styrene (ABS), are key components in enhancing antimicrobial, antioxidant, and biodegradable properties, as well as UV radiation stabilization, in the synthesis of composites or bio-composites through 3D printing.
Synthesis of lignin based polyurethane elastomers and films: The conventional methods of synthesizing LPUes often involve the use of hazardous or carcinogenic solvents, posing challenges in their removal. A solvent-free (SF) synthesis approach to produce lignin-containing polyurethane elastomers (SF-LPUs) exhibits remarkable toughness, elasticity, and strength. LPU elastomers derived from low molecular weight alkali lignin demonstrate exceptional elongation, good tensile strength, and a high elastic recovery ratio of 98.7 per cent.
Non-isocyanate-based polyurethane films (NIPU): A series of environmentally friendly non-isocyanate-based polyurethane films (NIPU) have been developed via a polycondensation reaction between diamine derivatives and biscyclic carbonate. The synthesis of biscyclic carbonate was achieved through the thiolene click and transcarbonation reactions of polyethylene glycol diacrylate and 1-thioglycerol.
Sustainable bio-PU: Fully harnessing the potential of high-performance lignin-based PU remains challenging, particularly in achieving a balance between reprocessability and excellent mechanical properties. A novel approach involving the reaction of phenolic lignin (PL) with isocyanate to create dynamic phenolic-carbamates, aiming to produce robust and reprocessable bio-PU elastomers is reported.
Self-healing LPU Elastomer: Self-healing coatings materials have a wide application in various fields, but achieving a high self-healing property and efficiency at ambient temperature without any additional power is still a big challenge. Since lignin contains a variety of active functional groups, such as aromatic groups, alcoholic hydroxyl groups, phenolic hydroxyl groups, etc., it can participate in chemical reactions such as esterification, oxidation, and reduction, which determines that it can replace polyols to prepare various types of polymers. LPUE was successfully prepared via partially substituting reactive raw material, chain extender, and filler with lignin.
Biological Potential of Chemically Modified Lignin
The use of natural and renewable raw materials such as lignin, which is primarily composed of phenylpropanoid that possesses a huge number of hydroxyl (OH) groups, phenols, and carbonyls has a potential to replace petroleum-based polyols in PU synthesis. The biological potential of chemically modified lignin shows better antibacterial, antioxidant, biocompatibility, flame retardancy, hydrophobicity, and UV-blocking properties. Lignin based polyurethanes have potential applications in construction, automated industries, packaging, textiles, footwear, supporting goods, automobiles, printing rollers, sealants, and binders.
With approximately 75 million tonnes of lignin generated annually as a byproduct of the pulp and paper industry, its utilization is expected to increase significantly in the future, particularly with the projected rise in renewable chemicals and fuels derived from lignocellulosic materials. Lignin, a naturally occurring polymer rich in reactive groups such as aromatic and aliphatic carbonyls, phenols, and hydroxyls, can be further enhanced through chemical modifications like depolymerization and oxyalkylation to improve the hydrophobic and thermal properties of PUs.
LPU synthesis predominantly employs the pre-polymerization method, involving polyester polyols or polyethers, chain extenders, polyisocyanates, functional additives, and crosslinking agents in preparation. Additionally, the use of polyols derived from lignocellulosic chain extenders can minimize PU fracture toughness, with lignin concentration significantly impacting PU characteristics.
Subscribe to our newsletter & stay updated.